MTaI statement on suspension of imports of refurbished devices

The government has suspended all imports of refurbished medical devices until the Ministry of Health and Family Welfare (MoHFW) establishes a policy framework for such imports. The pre-owned medical equipment market currently constitutes around 10% of the total medical equipment industry in India, playing a critical role in meeting the growing demand in Tier II, III, and IV cities, as well as rural and underserved areas.
These regions, where 70% of healthcare facilities are privately run small clinics, nursing homes, standalone hospitals, and diagnostic centers, rely heavily on cost-effective solutions.
Globally, even developed regions like the US and EU have a 7%-9% dependency on pre-owned medical equipment, underscoring its importance in ensuring affordable healthcare solutions. Countries like the US, several European nations, Japan, the UK, Canada, South Korea, New Zealand, and Australia permit the import and sale of pre-owned medical equipment by their regulations.
The Pre-owned equipment industry in India is valued at 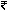 1,500 crore and supports the livelihoods of thousands of employees across its ecosystem. If a swiftly implementable policy is not introduced soon, it could lead to irreversible damage to both healthcare providers and the workforce. In this context, the expert committee must prioritize this matter and expedite its decision. Currently, the import of refurbished devices remains at a standstill, posing a threat to both the healthcare system and the industry itself.
1,500 crore and supports the livelihoods of thousands of employees across its ecosystem. If a swiftly implementable policy is not introduced soon, it could lead to irreversible damage to both healthcare providers and the workforce. In this context, the expert committee must prioritize this matter and expedite its decision. Currently, the import of refurbished devices remains at a standstill, posing a threat to both the healthcare system and the industry itself.
To safeguard the interests of the healthcare system and the refurbished medical device sector, the expert committee must expedite the creation of this policy and meanwhile to avoid any disruptions allow imports through necessary approvals by DGHS and MoEFCC.

